URI
URI Label
Revisions
Add to List
Edit Entry
Export:
XML
- November 2017 (5)
- October 2017 (6)
- March 2017 (1)
- November 2016 (11)
- October 2016 (3)
- September 2016 (3)
- August 2016 (4)
- July 2016 (1)
- June 2016 (2)
- March 2016 (1)
- February 2016 (1)
- January 2016 (1)
- December 2015 (8)
- Data (11)
- Experimental (33)
- Summary (3)
Show/Hide Keys
Daraprim: Summary for 2015
Overview
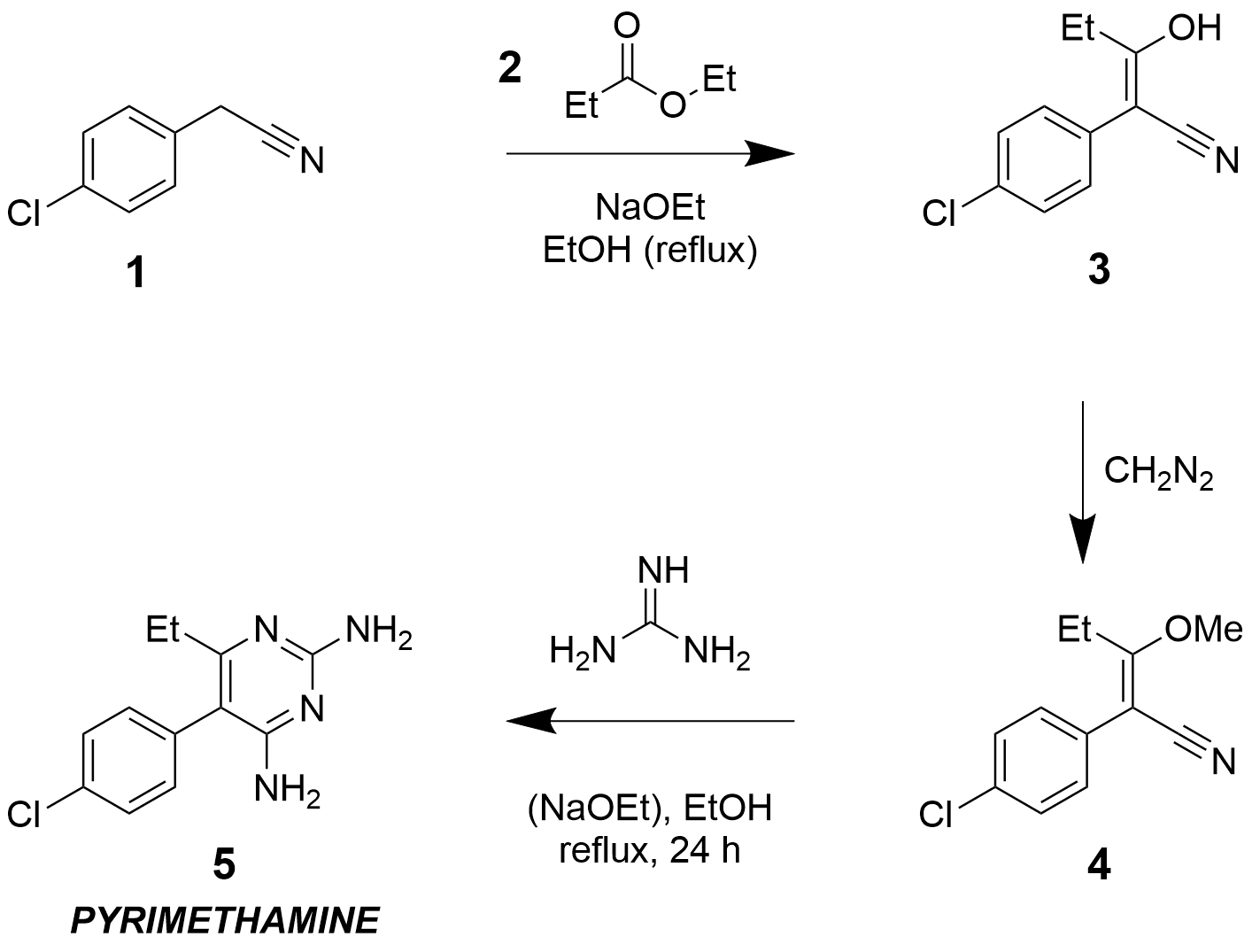
The historic synthetic routes1,2 to pyrimethamine (Daraprim) generally start with an p-chlorophenylacetonitrile 1. This is condensed with ester 2 under strongly basic conditions to give 3 (tautomeric with a stabilised enol form), which is then methylated to give enol ether 4. This alkylation has most commonly been performed using toxic and explosive diazomethane1,3; something that should certainly be avoided in teaching laboratory classes. Another possible alkylation method uses orthoformates4,5, which are significantly less hazardous. However, other literature reports indicate that these conditions are not effective at alkylating the enol.
Finally, pyrimethamine 5 is obtained from the enol ether 4 by condensation with free guanidine in refluxing ethanol. As guanidine is strongly basic (the guanidinium ion being strongly resonance stabilised), the reaction mixture must first be prepared from a guanidine salt and an alkoxide base. Most of the literature reports involve dissolving sodium into ethanol to form sodium ethoxide, followed by addition of guanidine hydrochloride to form free guanidine. This mixture is reported to be very air sensitive, as dissolved atmospheric CO2 will quickly form the guanidinium carbonate.
In summary: the literature route involves three reaction steps, of which two require alkoxide bases, and one generally involves diazomethane. Each of these classes of reagents would be challenging to use in a teaching laboratory.
Attempted route to access pyrimethamine
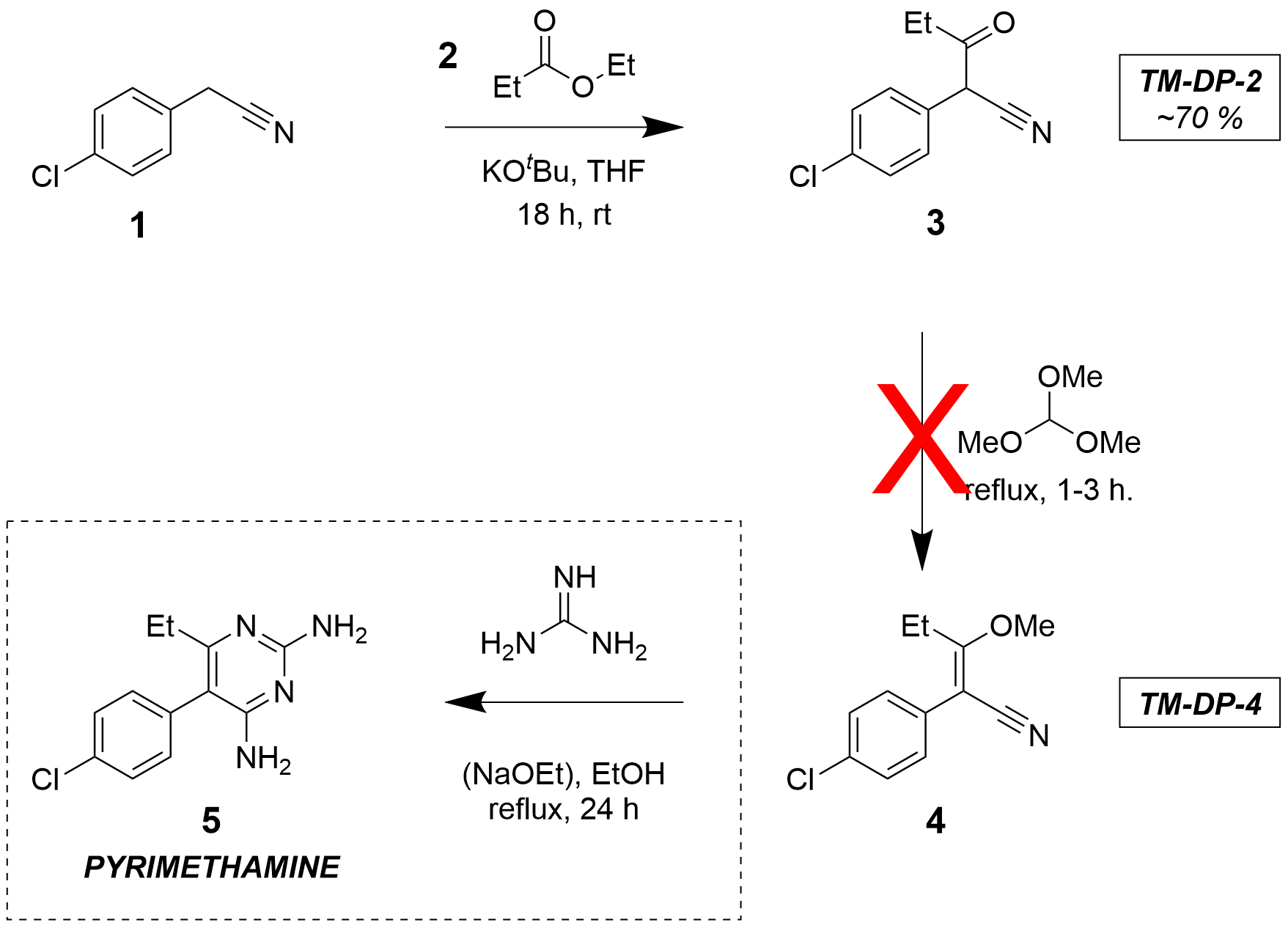
The route shown above was proposed in an attempt to avoid some of the risks associated with the historical route. Good yields of TM-DP-3 (3) were achieved via a KOtBu-mediated coupling at room temperature. Despite being a stronger base, this was judged less hazardous than using NaOEt as KOtBu allows the reaction to be conducted at room temperature (rather than in refluxing ethanol).
Two other reaction conditions were applied in attempts to produce 3: NaOMe in refluxing ethanol, and KOH + catalytic 18-crown-6 in refluxing toluene. Neither reactions were successful.
With 3 obtained in good yield, a number of attempts were made to form the enol ether 4 by alkylation with trimethyl orthoformate. None of these were successful.
Reaction 1 – Acyl condensation
Sodium methoxide (ethoxide was unavailable) in refluxing ethanol:
Synthesis of (Z)-2-(4-chlorophenyl)-3-hydroxypent-2-enenitrile - TM-DP-1-1
Unsuccessful.
Potassium tert-butoxide in room-temperature THF:
Synthesis of 2-(4-chlorophenyl)-3-oxopentanenitrile - TM-DP-2-1
Repeat synthesis of 2-(4-chlorophenyl)-3-oxopentanenitrile - TM-DP-2-2
Repeat synthesis of 2-(4-chlorophenyl)-3-oxopentanenitrile - TM-DP-2-3
Successful. Yield 68% at 12.5 mmol scale.
Potassium hydroxide with catalytic 18-crown-6 in refluxing toluene:
Synthesis of 2-(4-chlorophenyl)-3-oxopentanenitrile using KOH and 18-crown-6 - TM-DP-3-1
Unsuccessful.
Reaction 2 – Enol alkylation
Attempted alkylation with trimethyl orthoformate
Synthesis of 2-(4-chlorophenyl)-3-hydroxypent-2-enenitrile - TM-DP-4-1
Due to time limitations, the crude material was carried forward without characterisation.
Reaction 1 – Guanidine condensation
Synthesis of 5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine - TM-DP-5-1
Appears unsuccessful.
References
(1) Russell, P. B.; Hitchings, G. H. J. Am. Chem. Soc. 1951, 73 (8), 3763–3770.
(2) 2:4-DIAMINOPYRIMIDINES—A NEW SERIES OF ANTIMALARIALS - FALCO - 2012 - British Journal of Pharmacology and Chemotherapy - Wiley Online Library (accessed Nov 9, 2015).
(3) El-Hamamsy, M. H. R. I.; Smith, A. W.; Thompson, A. S.; Threadgill, M. D. Bioorg. Med. Chem. 2007, 15 (13), 4552–4576.
(4) Russell, P. B.; Whittaker, N. J. Am. Chem. Soc. 1952, 74 (5), 1310–1313.
(5) Swaringen, R. A.; Yeowell, D. A.; Wisowaty, J. C.; El-Sayad, H. A.; Stewart, E. L.; Darnofall, M. E. J. Org. Chem. 1979, 44 (26), 4825–4829.